Description
Supplement First is an Authorized Retailer for Integrative Therapeutics.
- Integrative Therapeutics V Clear EPs 7630™is an upper respiratory treatment containing a proprietary, homeopathic preparation of Pelargonium sidoides extract which addresses the underlying cause of symptoms to help speed recovery, and shorten the duration of upper respiratory irritations.
- EPs 7630 is a proprietary extract which has been the subject of over 20 clinical studies involving more than 9000 patients. Over 3,800 patients have participated in controlled double-blind studies and over 5,400 patients in open-label and non-interventional studies. It has been an effective, well-tolerated, leading European medicine for more than a decade.
- Exclusive, clinically proven extract with more than 20 clinical studies, including 9 prospective, randomized, double blind, parallel group, placebo-controlled clinical trials.
- Shortens duration and reduces severity of upper respiratory tract irritations
- Studied in more than 9,000 patients
- Shown to be well-tolerated in clinical studies
- Available in original drops or 99.9% alcohol-free cherry flavored syrup
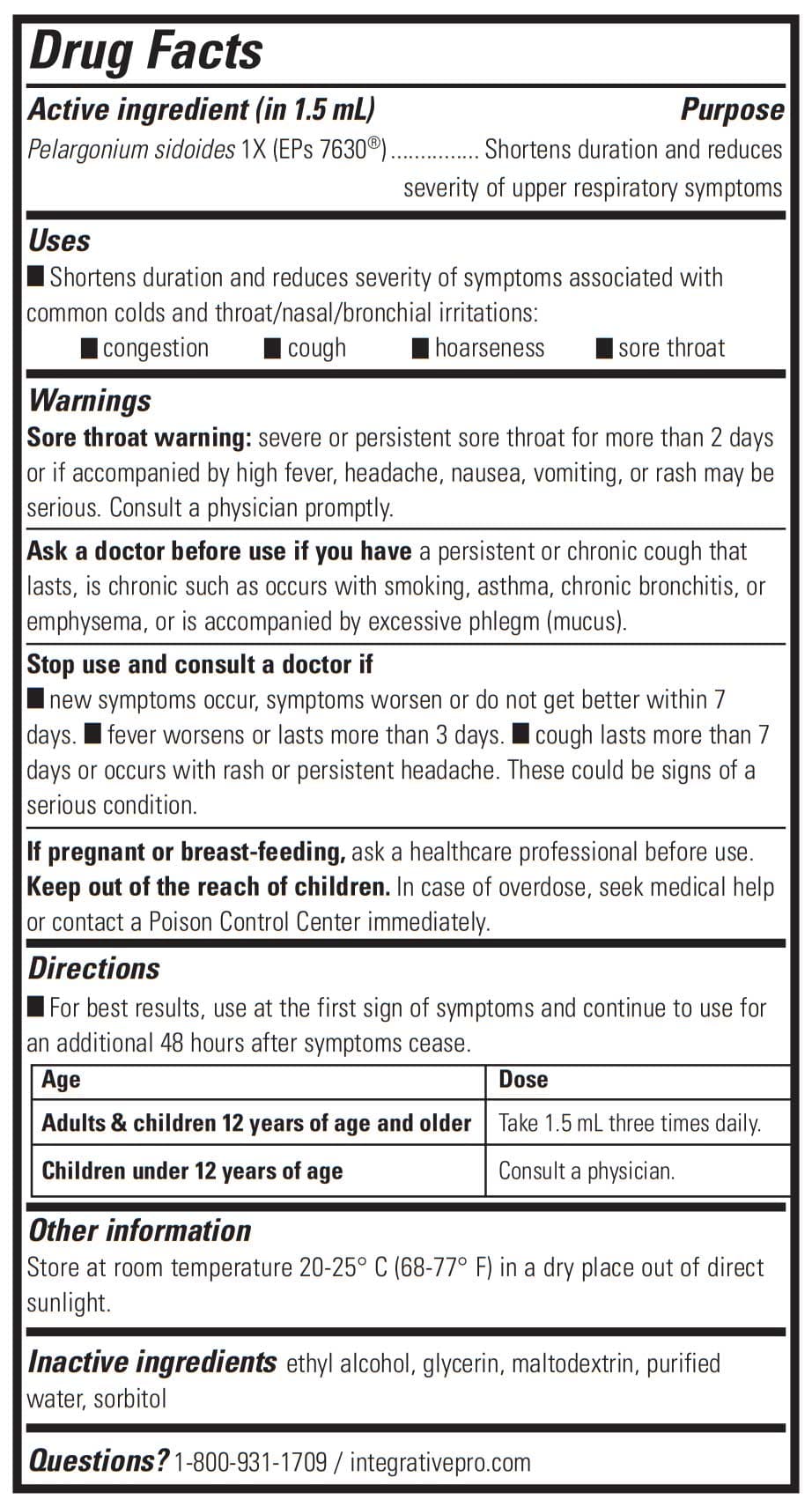
Original Flavor Drug Facts
Active Ingredient (in 1.5 mL)
Pelargonium sidoides 1X (EPs 7630®)
Purpose: Shortens duration and reduces severity of upper respiratory symptoms
Inactive Ingredients: Ethyl alcohol, glycerin, maltodextrin, purified water, sorbitol.
Suggested Use: For best results, use at the first sign of symptoms and continue to use for an additional 48 hours after symptoms cease. Adults & children 12 years of age and older: Take 1.5 mL three times daily. Children under 12 years of age: Consult a physician.
Warnings: Sore throat warning: severe or persistent sore throat for more than 2 days or if accompanied by high fever, headache, nausea, vomiting or rash may be serious. Consult a physician promptly. Ask a doctor before use if you have a persistent or chronic cough that lasts, is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema or is accompanied by excessive phlegm (mucus)
Stop use and consult a doctor if new symptoms occur, symptoms worsen or do not get better within 7 days. Fever worsens or lasts more than 3 days. Cough lasts more than 7 days or occurs with rash or persistent headache. These could be signs of a serious condition. If pregnant or breast-feeding, ask a healthcare professional before use. Keep out of the reach of children. In case of overdose, seek medical help or contact a Poison Control Center immediately.
* These statements have not been evaluated by the U.S. Food and Drug Administration. This product is not intended to treat, mitigate, diagnose or cure any disease.