Description
- Allergy Research Group Glucose Tolerance II provides glucevia, a patented european ash extract which safely supports blood sugar within normal levels.* With berberine, chromium, milk thistle, resveratrol, and biotin.
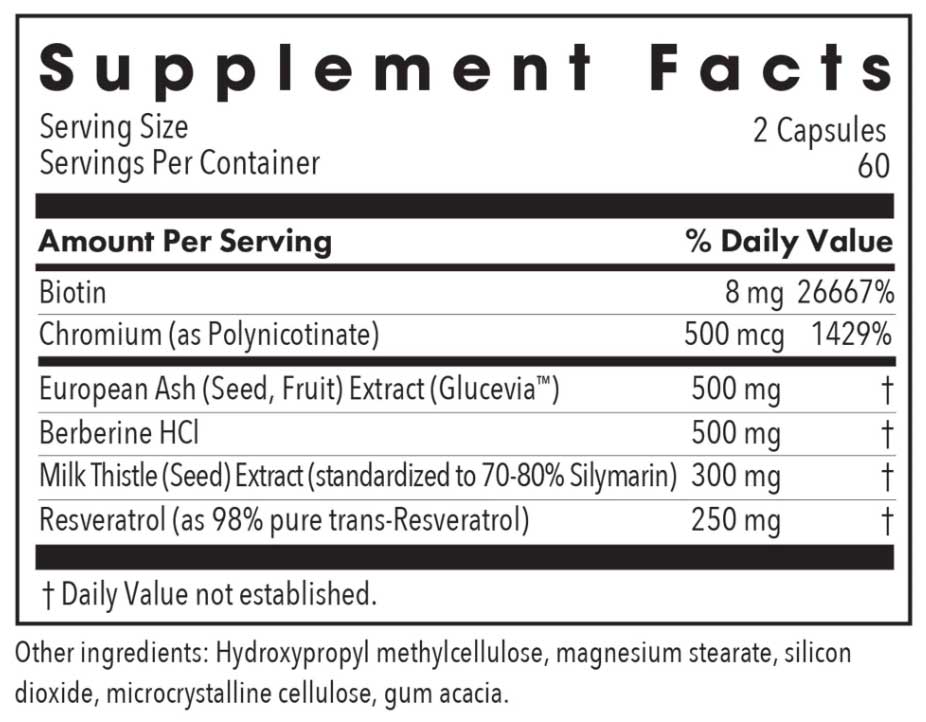
Supplement Facts
| Serving Size: 2 Capsules | Amount Per Serving | %DV |
| Biotin | 8 mg | 26667% |
| Chromium (as Polynicotinate) | 500 mcg | 1429% |
| European Ash (Seed, Fruit) extract (Glucevia™) |
500 mg | † |
| Berberine HCl | 500 mg | † |
|
Milk Thistle |
300 mg | † |
| Resveratrol (as 98% pure trans-Resveratrol) | 250 mg | † |
†Daily Value not established.
Other Ingredients:
Hydroxypropyl methylcellulose, magnesium stearate, silicon dioxide, microcrystalline cellulose, gum acacia.
Suggested Use:
As a dietary supplement, take 2 capsules twice daily with meals, or as directed by a healthcare practitioner.
Warning:
If you are pregnant or lactating, have any health condition or are taking any medication, consult your healthcare practitioner before use. Store in a cool, dry place, tightly capped, away from light. Keep out of reach of children. Use only if safety seal is intact. Variations in product color may occur.
Caution:
Biotin may interfere with certain blood tests. Wait at least 8 hours between biotin consumption and blood testing. Inform your healthcare practitioner about all biotin-containing supplements you are taking.
*These statements have not been evaluated by the U.S. Food and Drug Administration. This product is not intended to treat, mitigate, diagnose or cure any disease.